
Designated Marketing Authorization Holder (DMAH) Services
ISSUES
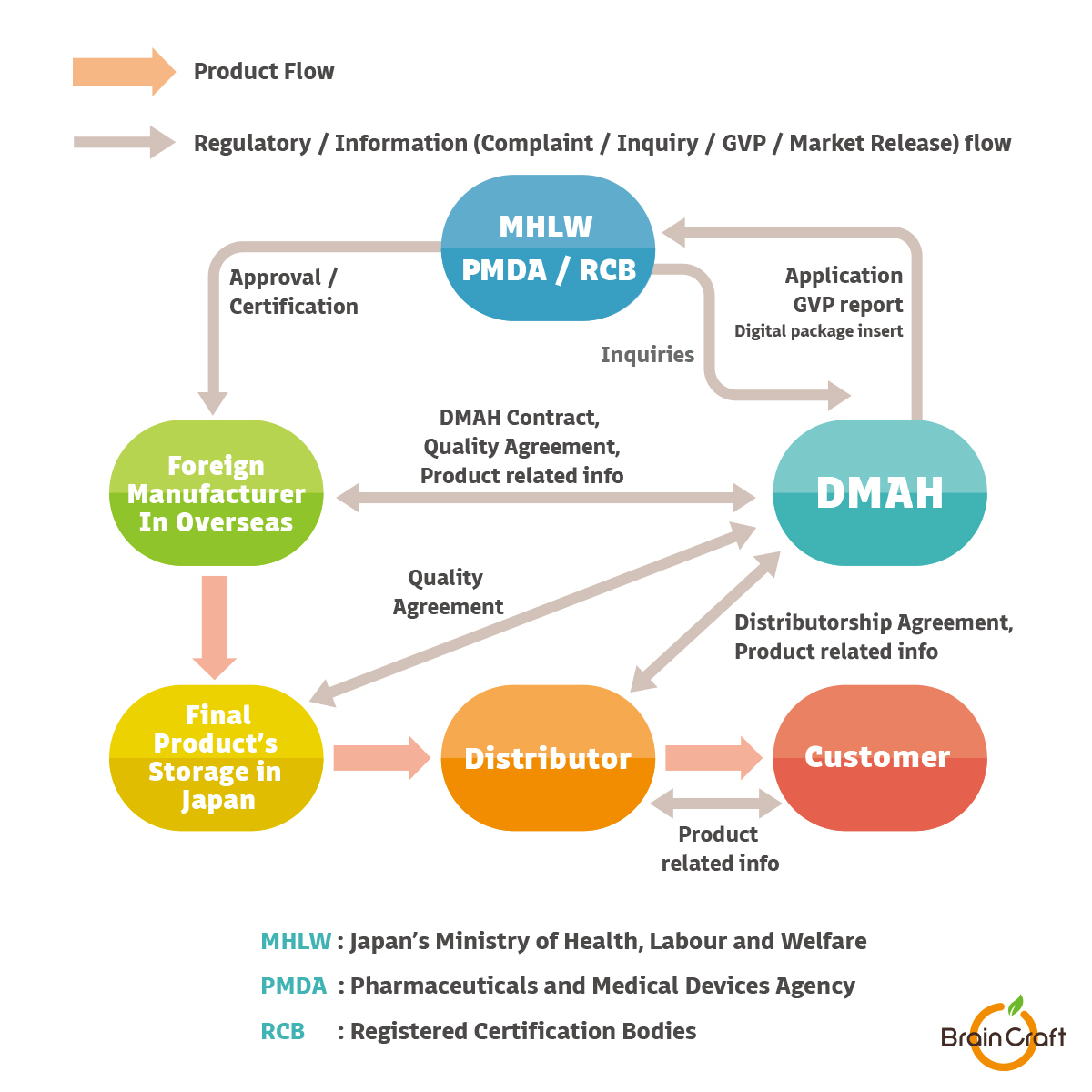
All establishments or facilities involved in the design, main assembly, final product storage or distribution of medical devices intended for use in Japan must have an appropriate business license or registration obtained according to the Japanese Pharmaceutical Medical Devices Act. from MHLW (Ministry of Health, Labour and Welfare).
Additionally, all medical devices marketed in Japan must be approved with MHLW in association with the Marketing Authorization Holder (MAH) or Designated Marketing Authorization Holder (DMAH) who is responsible for QMS and post-market safety control.
Foreign manufacturers with no business establishments in Japan must appoint a MAH or a DMAH to manage their device registrations.
SOLUTION
BrainCraft provides professional and independent Designated Marketing Authorization Holder (DMAH) services for Class I ~ IV Medical Devices.
We have the 1st grade Marketing Authorization Holder (MAH) license under the Japanese Pharmaceutical and Medical Device Act (PMD Act).
Designated Marketing Authorization Holder (DMAH) Services
| ・ | International Import/Export Issues |
| ・ | Japanese Agent for Foreign Medical Device Companies |
| ・ | Establishment of Quality Management System |
| ・ | FMR (Foreign Manufacturer Registration) registration |
| ・ | Registration of product catalogue numbers in EAN/GS1 codes |
| ・ | Regulatory application to reimbursement documents for health insurance for each category |
| ・ | Preparing products regulation label |
| ・ | Correction / Medical Device Incident Report |
| ・ | Device Master Record (DMR) |
| ・ | Internal Audit / Supplier Management |
|
Please contact us directly at info@braincraft.co.jp
or through the form under the CONTACT section of this site.










